SAFs Taking Flight
A deep dive into the technology of sustainable aviation fuels (SAFs) and how exactly they’re better
a carbon dioxide (CO2) molecule out in the wild
Rahul, what the heck is an SAF? Why should I care?
Great question to kick us off!
Sustainable aviation fuels (SAFs) are a type of fuel based on feedstocks that are renewable and importantly not petroleum-based. All conventional jet fuels (as well as gasoline and diesel) are derived from petroleum, which is problematic for two reasons:
Petroleum is not renewable! We’ll run out of it someday. We don’t know when exactly that will be, but petroleum doesn’t regenerate itself. The additional geo-political implication here is that reliance on petroleum means reliance on the specific countries that have it, both in terms of diplomacy and economics (with the ongoing war in Ukraine as a recent example).
It takes a lot of energy to process petroleum into jet fuel! This energy cost is accrued due to pumping the petroleum out from deep within the ground, transporting it to refineries, processing it to extract specific fuel molecules (through intense processes like fractional distillation), and then purifying this resulting mixture to meet jet fuel standards. All of these take energy and generate CO2 emissions in the process.
Conventional jet fuel use in aviation accounts for >2% total global greenhouse gas emissions and >12% in transportation specifically (source: U.S. DOE). SAFs have the potential to lower the total emissions of conventional jet fuel down by a whopping 94%! In addition to government-specified usage targets, this makes SAF a key technology for the near future.
Airline are now catching onto this trend as well. As a frequent flier out of San Francisco International Airport (SFO), a United Airlines hub, I’ve noted that the company has taken an assertive stance on incorporating more sustainable aviation fuels (SAFs) in their operations. This is not an endorsement for United Airlines, but it’s worth noting that when a legacy U.S. airline company starts talking about adopting new technology, you know that something is afoot.
What is the difference between conventional jet fuel and SAF?
Jet A fuel is the most common type of conventional jet fuel used in the United States. Jet A fuel is exactly specified by a standard (ASTM D1655), but the key is that it is kerosene-based, which is a combustible chemical derived from petroleum/oil and used in fuel applications worldwide. Kerosene consists of large molecules of hydrocarbons, which are molecules that consist of the elements hydrogen (“hydro-”) and carbon (“-carbon”) bonded together. These molecules are arranged in a chain of carbons, each with 2+ hydrogen atoms attached to them (see image). As a result, it’s common to refer to specific molecules primarily by the number of carbon atoms in the chain (e.g. “14 carbon chain” or even just “C14”). Almost all of the world’s power (excluding renewable sources) comes from physically burning hydrocarbon molecules and harnessing the energy from that reaction. Kerosene, as a result, contains larger hydrocarbons mostly in the C10-C16 size range, because larger molecules yield even more energy due to having more chemical energy in bonds that gets released when burned. Petroleum = kerosene = hydrocarbons = energy, end of story.
Examples of hydrocarbons with varying chain lengths
(source: Wikimedia Commons)
SAF is similar to Jet A fuel, but its origin is different. As mentioned earlier, Jet A fuel is derived from petroleum, which itself was turned into useful hydrocarbons over the course of millions of years underground by heat and pressure. SAF, however, comes from a variety of other places, namely other sources of molecules with lots of carbon that are not petroleum based. It turns out that hydrocarbons are abundant in things like food waste, woody biomass, algae, and other types of fats/greases/oils. While these feedstocks require further processing before they can be used as fuel, the fact that they are readily available and renewable (as opposed to petroleum that needs to get pumped and then come in via a pipeline and/or oil tanker) already starts to incur energy savings.
Are you saying that SAF feedstock is just as good as petroleum? If so, why can’t I just take my food scraps and power up a Boeing 747?
This is actually the million dollar question, because the devil lies in the details. The processing required to go from a feedstock (e.g. municipal waste, biomass, etc.) to a usable fuel has several steps, but the key idea is that each step is notably less energy intensive than the Jet A analog and there are fewer steps overall.
Step 1: obtain and purify a carbon-rich feedstock
SAF already takes the lead here. Hydrocarbons are abundant in the natural products mentioned previously, though theoretically, any combination of hydrogen and carbon atoms can be used as a feedstock, though the energy input required to transform this feedstock into a usable product will vary. Among other items, the International Civil Aviation Organization’s (ICAO) list of possible feedstocks include soybean oil, corn, coconuts, sugar, and even used cooking oil (source: ICAO).
Some feedstocks are also harder to purify than others. The plethora of items that get thrown into a municipal waste pile, for example, will be significantly harder to rid of unwanted side products compared to a barrel of a singular food from a farm. Non-hydrocarbons have the potential to alter the reactions that take place by interfering with them outright or creating toxic products when included (e.g. gaseous sulfur or chlorine).
Used cooking oil is a viable SAF feedstock!
(source: Wikimedia Commons)
Step 2: convert the feedstock into usable fuel
Though SAF can originate from a variety of carbon-rich feedstocks, the final product is almost always kerosene-based. This step is where the SAF magic occurs, as there are several methods and setups that can be used to get from feedstock to fuel. The most common and scalable methods, however, a;; go through the following steps:
Dehydrate the feedstock to eliminate any residual moisture (which would otherwise interfere with subsequent reactions)
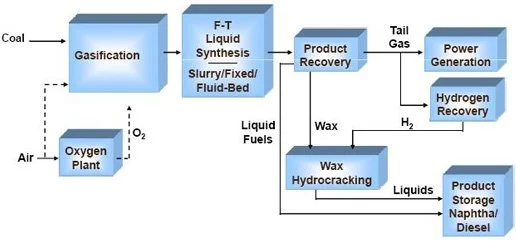
Under high temperature, add controlled amounts of oxygen and steam (H2O) to create a carbon monoxide (CO) + hydrogen gas (H2) mixture in a process known as gasification. This resulting mixture is known as “synthesis gas”, or syngas
Use the Fischer-Tropsch process, invented in the 1920s and in widespread use since World War II, to turn this syngas into kerosene products
Diagram of the full SAF process with Fischer-Tropsch (F-T) near the center
(source: U.S. Department of Energy)
Out of the sub-steps mentioned above, the gasification step is the most varied across the board. This process leads to partial oxidation of the feedstock: all this means is that controlled quantities of oxygen are supplied to the feedstock such that it reacts to form carbon monoxide (CO) and hydrogen (H2) as opposed to dousing the feedstock in oxygen which would result in burning along with significant carbon dioxide (CO2) formation. The latter undesirable process is considered to be total oxidation, but is more commonly referred to as combustion given the fact that visible burning usually takes place. This partial oxidation also allows the feedstock molecules to get broken down and releases even more heat, which then react to form the syngas products.
You can think of it similarly to toasting bread — you want to allow it to react with oxygen enough to get that brown crisp, but if you let it react with too much oxygen, you’re left with a blackened burnt piece of toast. You want the bread molecules to break down just enough to react with oxygen, but not too much that you ruin breakfast with burnt toast.
Gasification is like making crispy (but not burnt) toast!
(source: Wikimedia Commons)
The other sub-step is the Fischer-Tropsch (FT) process, which was invented in 1920s Germany and popularized after World War II. Though it has a complex-sounding name, all this process does is turn syngas into usable fuel under 150-300 °C and high pressure (as in 7-10x atmospheric pressure). These conditions (moderate heat and high pressure) promote the formation of long hydrocarbons (i.e. kerosene) from syngas. The FT process is highly exothermic, which means that it releases a lot of heat when run. As a result, reactors used to run FT need to be optimized to dissipate heat while maintaining a pressurized environment.
This process is already widespread and used across the globe, including in South Africa, which has large coal reserves but minimal petroleum or crude oil. Because coal is also a carbon-rich feedstock, countries like South Africa (lots of coal, not much petroleum) can use the FT process to create usable fuel to power not only planes but also other vehicles and devices.
A South African industrial plant that turns coal into liquid fuel using the FT process
(source: U.S. Department of Energy)
There are several other process options (often referred to as “production pathways” in both government-led reviews) that involve a wide variety of chemical engineering techniques, but at the end of the day, three things are true no matter which exact method you use:
INPUT: feedstock with hydrocarbons
OUTPUT: usable jet fuel
Energy will be CONSUMED going from input to output
Fischer-Tropsch reaction: syngas to fuel
(source: U.S. Department of Energy)
Wait, so you have to use energy to make SAF? How is that sustainable then if you’re putting energy in to make it?
Excellent point! You are correct that feedstock sourcing, gasification, and Fischer-Tropsch synthesis all require energy in order to proceed. The difference, however, is that each step for SAF production uses significantly less energy than the conventional jet fuel equivalent over the course of the full feedstock to usage life cycle (calculations are typically done through a life cycle analysis, which accounts for every emission from feedstock sourcing to burning during plane use). Through such life cycle analyses, it has been shown that using 100% SAF in a plane can reduce emissions by 94% compared to traditional jet fuel analysis, though exact figures will vary based on the pathway used (source: Prussi et al, 2021)
There are some general principles that can be used to help intuit how SAF uses less energy in each step than jet fuel:
Feedstock Sourcing: biomass/oils/greases can be found pretty much anywhere on Earth at surface level (SAF), though petroleum can only be mined in specific locations around the world and has to be pumped from deep within the ground back up to the surface
Feedstock Transport: biomass/oils/greases (SAF) are generally less dense than their petroleum counterparts. Furthermore, solids (such as SAF feedstock) are typically significantly easier to transport compared to liquid (like petroleum)
Feedstock Processing: this step is really where the most potential for difference can occur. Renewable feedstock goes through the reactions towards syngas and kerosene mentioned above (SAF), while petroleum goes through the energy intensive fractional distillation process (among others), which uses significant amounts of energy to refine petroleum. Even better, this step may be powered by renewable energy (like wind or hydropower) to truly approach carbon neutrality
Fuel Transport: once fuel has been created (either SAF or from petroleum), their densities are roughly the same, so the main variable is the distance fuel needs to travel to get to the airplane. Domestic American petroleum can flow through various oil pipelines to airports across the country, though internationally sourced oil comes in on tankers. The hope for SAF is that because feedstocks can likely be sourced locally, they will be processed locally as well, further reducing emissions in this step.
Though the exact figures for emissions reduction will vary on the exact pathway/chemistry used, there are technologies in development to reduce those figures further and even bring the entire process down to carbon neutral. At least one company, Sora Fuel (based in my native Cambridge, Massachusetts), is attempting to use air-captured CO2 as part of the SAF feedstock in order to bring it down further.
No matter what, one of the big advantages of SAF is the potential ability to use renewables to power the remaining intensive parts of the process for SAF.
Method for life cycle analysis of fuel emissions from feedstock to usage
(source: Prussi et al, 2021)
If you’re going to use renewable energy anyways then why can’t we just use those sources to directly power a plane?
Excellent question! If renewable energy can power a process to make jet fuel to power a plane, then why can’t we just power the plane directly? The answer entirely comes down to energy density, which is a measure of how much energy a given volume of a fuel can store. In short, the energy density of Jet A fuel is >25x than that of the most advanced airplane battery as of 2024 (source).
For aviation, a key fuel characteristic the ability to use the least weight while delivering maximum power. Several aviation developments have involved lightening existing materials in order to reduce the overall weight of an aircraft, though a significant source of the airborne weight is the dense fuel itself. If renewable energy is being used to directly power a plane, then the plane needs a battery to store all that energy and use it during flight.
Here’s the issue: jet fuel (~12,000 Watt-hours per kilogram) is >25x as dense energy-wise than the most advanced airplane battery (~450 Watt-hours per kilogram). To get more energy onboard an electric plane, you need to add more batteries, but you’ll have to add kilograms upon kilograms of batteries in order to meet the total energy required for flight. As a result, battery energy density needs to increase drastically before they can be used in electric flight. For now, SAF makes a lot more sense.
The Electric Airbus A320 is an example of a battery-powered aircraft
(source: Wikimedia Commons)
This sounds great and all, but why isn’t this everywhere? What’s next for SAF?
While production pathways are defined and also in deployment, the answer to why you’re not flying on 100% SAF fuel on all your flights comes to scale. There are just SO many more gallons of jet fuel required in the airline industry than gallons of SAF produced each year. United Airlines alone produces 10 million gallons of SAF each year, which is a lot, but barely makes a dent in the 4 billion gallons required annually by the airline alone (source: Wall Street Journal). This means that the order of ~0.1% of United’s jet fuel needs can even be satisfied with SAF right now.
Therefore, the largest challenge to SAF use is scaling up production. There are multiple challenges to this, including not only building enough SAF refineries but also ensuring the SAF production pathway results in the fewest carbon emissions possible. Lucky for us, plans to do just this are in action: a prime example is Montana Renewables LLC, which is the nation’s leader in SAF production with 30 million gallons made each year for several airlines. There a ton of technology development on the horizon to improve the tech further as well, including large players like Neste and smaller startups like Sora Fuel tuning new processes.
It’s exciting to see when the next batch of SAF-powered flights will come online!